About Us
Focusing on the continuous innovation of diagnostic and treatment technologies for sleep breathing disorders and respiratory diseases, Hibisure provides high-quality products and services for global users in the fields of pulmonary function testing, non-invasive ventilation, oxygen therapy, and inhalation drug delivery.
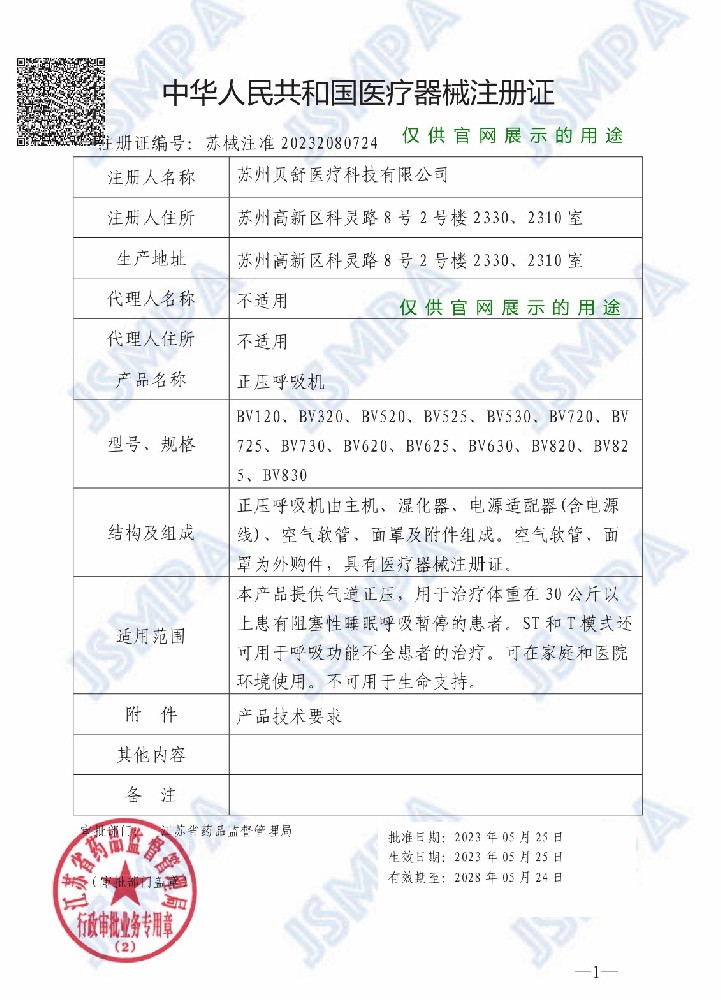
Headquartered in Suzhou Science and Technology City, Hibisure has set up two production bases in Wuzhong, Suzhou and Jiangning, Nanjing, with a complete R&D, production and service system and independent laboratories, manufacturing department and quality inspection department. Our products have successively obtained the registrations and certificates of NMPA, CE, and FDA, and we have also accumulated a number of patents, trademarks, and software copyrights around the globe.
Relying on BiSure call network health management system,Hibisure will carry out digital management of the whole disease process around the different stages of diagnosis, treatment and rehabilitation of respiratory diseases, and provide more humane technical services and health care for every user.
100+ 70,000 sqft 1,000,000+pics
Employees Manufacturing plant Annual sales